Creating Insights
Digi-HTA: adding value to digital health services assessment
Limited resources and the need to control costs cause challenges in healthcare. Digital health services enable reformation of processes and allocation of resources in a new way. Digital health services include solutions based on artificial intelligence (AI), IoT-technology, mHealth, and the use of robotics.
HTA is the evaluation of health technologies
New treatment methods are constantly introduced in healthcare and there are more and more alternative methods for treating various diseases. It is clear that information is needed when deciding on new healthcare methods. Health Technology Assessment (HTA) combines research evidence for objective and quality information that can be utilised in decision making. HTA is used for existing and new methods including medicines, procedures, and equipment. Health Technology Assessment is needed, in particular, if the health technology is new or exceptionally expensive.

What is digi-HTA?
Digi-HTA provides a systematic framework for assessing digital health services in different areas of healthcare. The aim is to evaluate and demonstrate the suitability of digital health services through scientific evidence. In addition, the objective is to facilitate healthcare decision making in terms of digital health services. Companies can also use the digital assessment tool for self-evaluation and value demonstration of their own digital services and products, as well as their development.
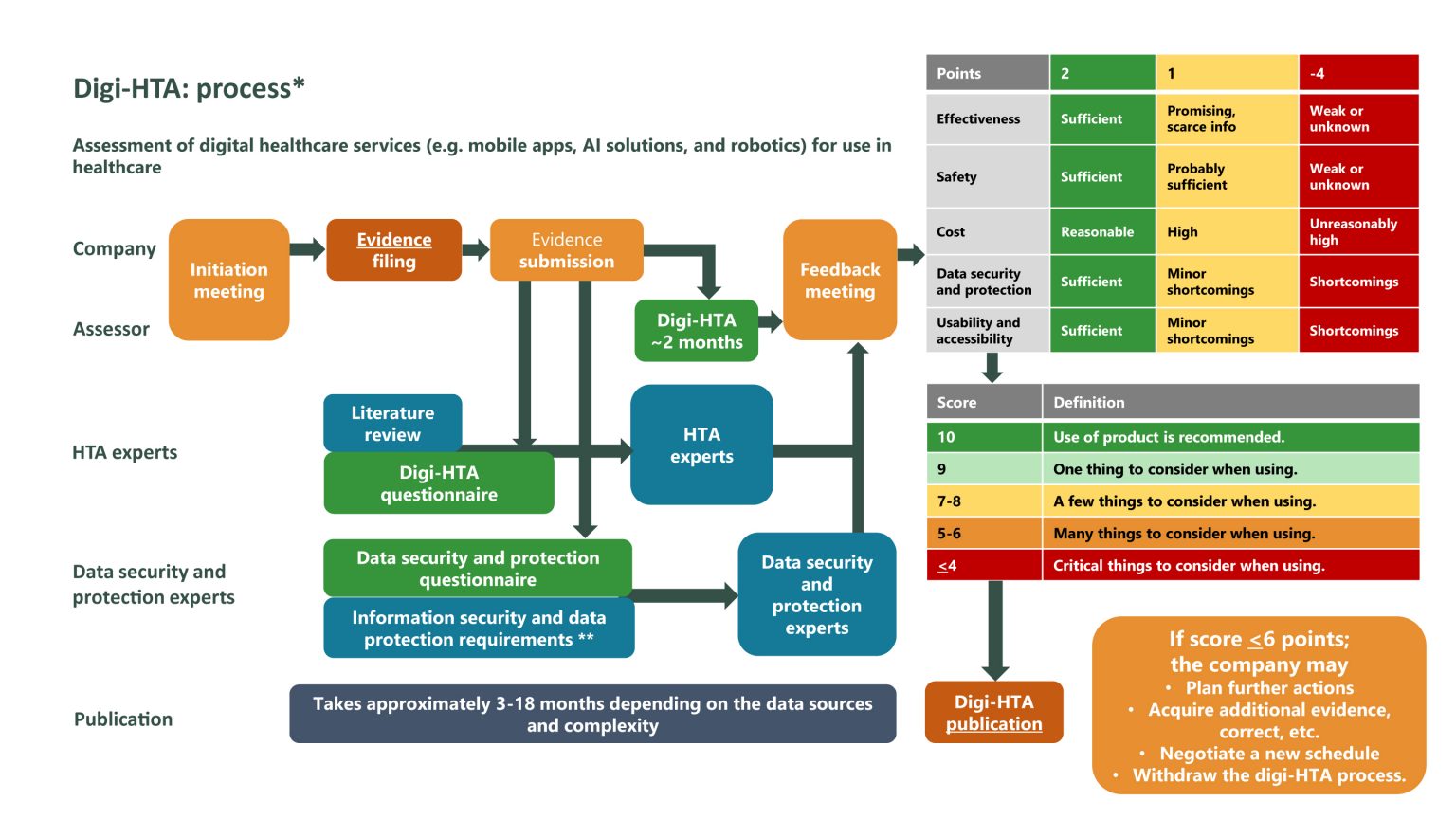
The digi-HTA process and criteria were developed in 2019 on behalf of the Finnish Ministry of Social Affairs and Health. The Faculty of Medicine of the University of Oulu and the national HTA coordination unit FinCCHTA created the assessment process. Digi-HTA is used to evaluate the suitability of a product or service in terms of effectiveness, security, cost, data security and data protection, usability, and accessibility. Digi-HTA is suitable for both medical and non-medical services and products. The assessment supplements the CE marking of medical devices, which indicates conformity with health, safety, and environmental protection standards for products sold within the EEA.
Finally, FinCCHTA will make an objective digi-HTA recommendation on the digital health product or service. All recommendations are published on the FinCCHTA website and a recommendation is usually valid for three years. Digi-HTA is becoming an established national process for digital health services in Finland and it can recommend the use of health technologies. The process is illustrated in the picture below.
How will ESiOR streamline the Digi-HTA process?
Digi-HTA can be requested from FinCCHTA. The requesting company will then provide information on its digital service or product to FinCCHTA. In practice, gathering and combining data (e.g. literature reviews and meta-analyses) and demonstrating cost-effectiveness can be big challenges. ESiOR offers solutions to these challenges with years of experience. We will help gain insight and demonstrate competitive advantage of health technology through data and analysis.
We will demonstrate costs, effectiveness, and cost-effectiveness as well as combine evidence and conduct meta-analyses from different data sources. In addition, we can strengthen the information on effectiveness with real world evidence (RWE). For example, register and survey studies can be used to provide additional evidence of the effectiveness and costs in everyday life. If needed, ESiOR can also provide a scientific publication to further support decision making and marketing.

Does your company provide digital health services or products?
Let’s create value with digi-HTA to support your customers’ purchase decisions!
https://thl.fi/fi/tutkimus-ja-kehittaminen/tutkimukset-ja-hankkeet/hyvinvoinnin-tekoaly-ja-robotiikka-ohjelma-hyteairo-/digi-hta (in English and Finnish)
https://www.ppshp.fi/Tutkimus-ja-opetus/FinCCHTA/Sivut/Digi-HTA.aspx (in Finnish)
Interested? Contact us and we will tell you more.
More information about ESiOR services on digi-HTA.
Digi-HTA contact
CEO Erkki Soini
+358 40 053 3971
Our email addresses are in the following format
firstname.lastname(at)esior.fi